作者简介:吕 游,在读本科生。
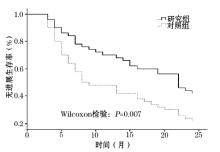
目的 探讨贝伐单抗联合曲妥珠单抗+紫杉醇(TH)方案化疗对人类表皮生长因子受体2(human epidermal growth factor receptor-2, Her-2)阳性的转移性乳腺癌患者外周血T细胞亚群和临床预后的影响。方法 选取Her-2阳性的乳腺癌患者100例,随机分为研究组和对照组;研究组采用贝伐单抗联合TH方案化疗,对照组仅采用TH方案化疗。随访终点为2年,主要观察指标为CD4+T细胞、CD8+T细胞、实体瘤疗效评价等级、无进展生存期和2年病死率。结果 两组治疗前后CD8+T细胞差异均无统计学意义。两组治疗前CD4+T细胞差异无统计学意义( P=0.422)。治疗后,研究组CD4+T细胞显著高于对照组( P=0.011)。研究组完全缓解、部分缓解、病情稳定和病情进展发生率分别为0.0%、36.0%、42.0%和22.0%,对照组为0.0%、18.0%、44.0%和38.0%。2年后,研究组9例死亡,病死率为18.0%,对照组20例死亡,病死率为40.0%,差异有统计学意义( P=0.015)。Wilcoxon检验显示研究组无进展生存期显著高于对照组( P=0.007)。结论 贝伐单抗联合TH方案化疗有助于改善Her-2阳性的转移性乳腺癌患者免疫功能和临床预后。
Objective To investigate the effect of bevacizumab combined with TH chemotherapy on T cell subsets in peripheral blood and clinical prognosis in human epidermal growth factor receptor 2 (Her-2) positive patients with metastatic breast cancer.Methods Between Jan, 2012 and Jan, 2014, 100 patients with Her-2 positive metastatic breast cancer were prospectively recruited in this study. These patients were randomly assigned into a study group or a control group. The study group was treated with bevacizumab combined with TH chemotherapy while the control group was treated with TH chemotherapy alone. The patients were followed up for two years.The primary outcomes included CD4+T cells, CD8+T cells, solid tumor efficacy evaluation grades, progression free survival and 2-year mortality.Results There was no significant difference in CD8+T cells between the two groups before and after treatment. After treatment, the expression of CD4+T cells in the study group (
乳腺癌是中老年女性发病率最高的恶性肿瘤[1, 2], 随着医疗技术的发展, 其病死率已明显降低[3, 4]。人类表皮生长因子受体2(Human epidermal growth factor receptor-2, Her-2)高度表达与肿瘤细胞的快速增殖和侵袭紧密相关[5]。Keyhani等[6]发现, Her-2阳性的乳腺癌发生率高达44.5%。对于Her-2阳性的乳腺癌, 初诊时部分患者已合并远处转移, 失去根治性切除的机会, 化疗是常用的治疗方法, 其中常用的方案为曲妥珠单抗联合紫杉醇, 即TH方案[7, 8]。贝伐单抗是一种血管内皮生长因子抑制药, 可以通过抑制肿瘤血管的生成而降低肿瘤细胞生长和转移的风险。本研究旨在探讨贝伐单抗联合TH方案化疗对Her-2阳性的转移性乳腺癌患者外周血T细胞亚群和临床预后的影响。
选取2013-01至2015-01武警后勤学院附属医院收治的Her-2阳性的乳腺癌患者100例, 随机分为研究组和对照组, 每组50例。研究组34~65岁, 平均(51.76± 6.37)岁, 浸润型导管癌38例, 小叶癌8例, 黏液腺癌4例; 32例为术后发现转移, 18例首诊即确定为转移, 28例为肺转移, 14例为肝转移, 8例为其他部位转移。对照组36~65岁, 平均(52.08± 5.88)岁, 浸润性导管癌41例, 小叶癌6例, 黏液腺癌3例; 28例为术后发现转移, 22例为首诊即确定转移, 31例为肺转移, 15例为肝转移, 4例为其他部位转移。两组年龄、肿瘤细胞组织学类型、转移部位等差异均无统计学意义。所有患者均知情同意并签署知情同意书, 通过我院伦理委员会批准。
(1)转移性乳腺癌(经术前穿刺或术中病理确诊, 同时合并有远处转移); (2)Her-2阳性; (3)年龄≥ 18岁且≤ 65岁; (4)同意参与本研究。排除标准:(1)合并其他恶性肿瘤; (2)6个月内曾发生心肌梗死、脑卒中、脑出血等严重心血管不良事件; (3)肝肾等脏器功能不全; (4)凝血功能障碍; (5)骨髓抑制; (6)甲状腺功能不全; (7)急性或慢性感染期; (8)研究期间转院、失访、不配合治疗或放弃治疗。
(1)研究组给予贝伐单抗联合TH方案化疗:曲妥珠单抗(美国基因科技公司, 批准文号:S20060026)首次剂量4 mg/kg, 后续2 mg/kg, 1次/周, 共7~14次。紫杉醇(哈药集团生物工程有限公司, 批准文号:国药准字H20059962)175 mg/m2, 第1天, 静脉点滴, 3周为一疗程。共2~4个疗程。贝伐单抗(美国罗氏生物制药公司, 批准文号:国药准字BS20067454)10 mg/kg, 1次/2周, 4周为一个周期。(2)对照组:仅给予TH方案, 用法同研究组。
观察比较两组化疗前、化疗开始后6个月时CD4+T细胞、CD8+T细胞、化疗开始后6个月时健康相关的生存质量(SF-36)、化疗开始后6个月时实体瘤疗效评价等级、化疗相关并发症、无进展生存期和2年病死率。实体瘤疗效评价等级:根据化疗后患者情况将疗效分为4个等级:(1)完全缓解:病灶消失4周以上; (2)部分缓解:病灶最大直径缩小30%以上; (3)疾病稳定:介于部分缓解和病情进展之间; (4)病情进展:病灶直径增加20%以上或出现新病灶。
应用SPSS 22.0软件, 不同疗效评价等级采用秩和检验; 两组无进展生存期的差异采用生存函数进行统计分析, P< 0.05为差异有统计学意义。
两组治疗前后CD8+T细胞水平差异均无统计学意义。两组治疗前CD4+T细胞差异无统计学意义, 治疗后研究组CD4+T细胞水平显著高于对照组(P=0.011, 表1)。
| 表1 两组Her-2阳性乳腺癌患者化疗前后外周血CD4+T细胞和CD8+T细胞水平比较(n=50; |
研究组和对照组部分缓解、病情进展发生率差异有统计学意义(P=0.025, 表2)。
| 表2 两组Her-2阳性乳腺癌患者化疗开始后6个月时实体瘤疗效评价等级比较[n=50; (n; %)] |
与对照组比较(67.37± 9.88), 研究组健康相关的生存质量(SF-36)评分(72.57± 12.58), 显著增加(P=0.024)。两组末梢神经炎、胆红素升高、中性粒细胞减少症、白细胞减少症和肠穿孔或出血发生率无统计学差异(表3)。
| 表3 两组Her-2阳性乳腺癌患者并发症比较[n=50; (n; %)] |
Her-2阳性的乳腺癌患者总生存期仅为62个月[9]。贝伐单抗是一种人源性单克隆抗体, 因为其具有抑制血管内皮生长因子的作用, 因此被临床上广泛用于治疗癌症, 它可抑制肿瘤血管的形成, 最终抑制肿瘤细胞的生长和转移。但是, 贝伐单抗致肠道出血、穿孔等并发症发生率较高, 在肿瘤治疗中的安全性和有效性存在争议。鉴于Her-2阳性的转移性乳腺癌病死率高、生存期短, 有学者开始尝试联合使用贝伐单抗和TH方案治疗Her-2阳性的转移性乳腺癌。黄仕思等[10]回顾性分析了88例Her-2阳性的转移性乳腺癌患者, 其中45例联合应用贝伐单抗和TH方案治疗, 43例单独采用TH方案治疗。结果显示, 贝伐单抗联合TH方案治疗显著改善了Her-2阳性的乳腺癌患者生存期, 且患者对贝伐单抗联合TH方案化疗耐受性较好, 未出现严重的不良反应。但由于该研究为回顾性临床研究, 因此该作者指出尚需要大样本量、多中心的随机对照研究证实。本前瞻性研究显示, 贝伐单抗联合TH方案显著降低了Her-2阳性的转移性乳腺癌患者2年病死率, 延长了无进展生存期, 并改善了生存质量和免疫功能。然而, 目前关于贝伐单抗在乳腺癌患者中应用的安全性和有效性仍是有争议的, 2013年Kader等[11]发现, 贝伐单抗联合化疗无助于改善患者生存期, 但显著增加了贝伐单抗相关性高血压发生率。2013年Stark等[12], 贝伐单抗无益于改善患者临床预后, 但导致患者健康相关的生存质量评分显著降低[(71.28± 17.60) vs (66.80± 15.23), P=0.228]。本研究显示, 贝伐单抗联合应用TH方案化疗, 显著提高了患者健康相关的生存质量, 仅1例患者出血肠道出血, 经过非手术对症支持治疗后好转, 并无其他严重并发症, 且显著延长了患者无进展生存期, 降低了患者2年病死率。由此可见, 目前关于贝伐单抗联合TH方案对于Her-2阳性的转移性乳腺癌预后影响的研究较少且结论不一, 仍需要大量的多中心、随机对照研究予以进一步验证。
肿瘤细胞的转移与机体免疫功能息息相关, 乳腺癌患者可表现为Th1/Th2细胞免疫向Th2细胞免疫漂移, 且这种免疫抑制严重程度与乳腺癌患者疾病严重程度成正比[13, 14, 15]。CD4+T细胞在炎性刺激下可以分化为Th0细胞, 进一步可以分化为Th1和Th2细胞。乳腺癌患者外周血中CD4+T细胞水平往往降低[16]。本研究显示, 贝伐单抗联合TH方案化疗显著提高了患者外周血CD4+T细胞水平, 有助于改善乳腺癌患者免疫功能, 但具体机制尚不清楚, 可能与贝伐单抗抑制肿瘤的细胞的生长和转移, 进而减弱了乳腺癌患者肿瘤细胞对机体免疫功能的打击。
总之, 贝伐单抗联合TH方案化疗应用于Her-2阳性的转移性乳腺癌患者是安全的, 可能有助于改善患者免疫功能和临床预后。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|